
Raimund Oberle, PhD
Molecular BiologistTel.: +43 (0)1 40160-38022
E-Mail: raimund.oberle@meduniwien.ac.at
ORCID: https://orcid.org/0000-0001-6701-4130
Tumor immunosuppression and lipid metabolism
Epidemiological data demonstrate that patients suffering from obesity and the metabolic syndrome have a higher risk to develop cancer. Obesity-associated metabolic perturbations foster tumor growth and progression by suppressing anti-tumor T cell activity and promoting a highly tumor-permissive microenvironment. Myeloid-derived suppressor cells (MDSC) are a heterogeneous group of pathologically activated neutrophils and monocytes with immunosuppressive character that are predominantly present in patients suffering from cancer. Soluble, tumor-secreted cytokines such as M-CSF, GM-CSF, VEGF and IL-6, amongst others, stimulate aberrant myelopoiesis in the bone marrow, thereby leading to the expansion of MDSC and their recruitment into the tumor microenvironment. Once residing in the tumor, MDSC efficiently suppress anti-tumor T cell responses via amino acid depletion, reactive oxygen species production and the secretion of immunomodulatory cytokines, amongst others. Interestingly, previous research identified MDSC and tumor-associated macrophages as major drivers of obesity-associated immunosuppression in preclinical tumor models.
By using syngeneic murine tumor models, biochemical and functional cell biological methods as well as immunological assays, we try to understand how obesity-associated alterations in systemic lipid and glucose metabolism affect the immunosuppressive behavior of myeloid cells in the TME. Specifically, we aim at the identification of novel mechanisms that drive the activation and maturation of MDSC in the TME, thereby acquiring an immunosuppressive phenotype resulting in efficient inhibition of the anti-tumor T cell response.
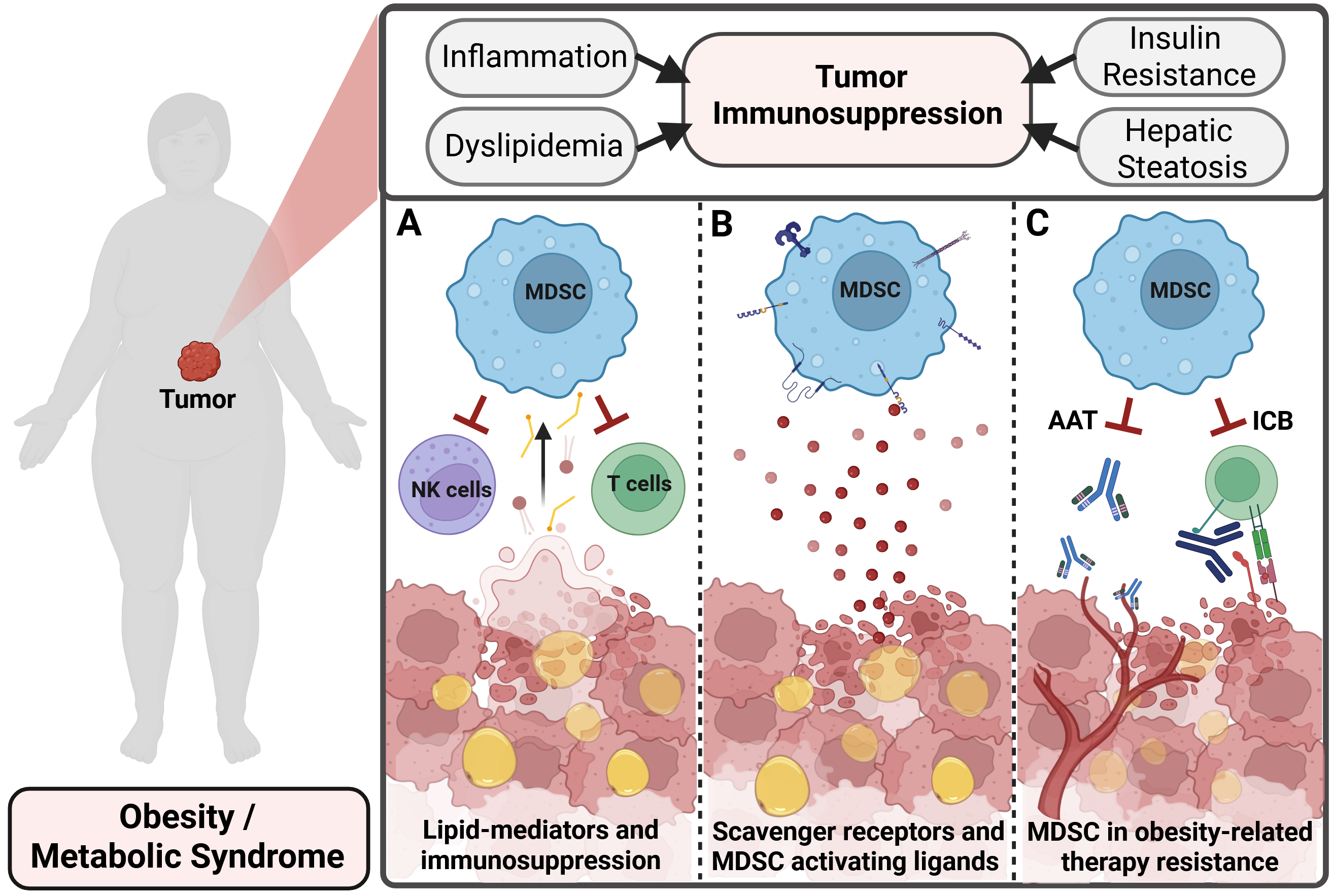
Obesity and the Metabolic Syndrome affect various aspects of tumor immunosuppression. Specifically, we are interested in A) identifying lipid associated metabolic pathways that foster the immunosuppressive action of MDSC; B) detecting potential novel, obesity-associated activating ligands in the TME that engage MDSC-specific cell surface receptors; and C) unraveling MDSC-centered mechanisms that hinder the therapeutic efficacy of anti-angiogenic – and immunotherapy. Figure created in https://BioRender.com
Lipid mediators and immunosuppression. Obesity is associated with increased tumor burden and an immunosuppressive microenvironment. We use tumor models in mice with obesity and aim to uncover novel, MDSC-centered mechanisms that suppress the anti-tumor immune response. Thereby, we focus on obesity-associated metabolic changes that promote MDSC suppressive activity.
Ligands of MDSC in the TME. Once homing into the TME, MDSC are activated and suppress anti-tumor T cell activity. We apply a biotin-ligase-based proximity labeling approach to identifiy novel, scavenger receptor-specific ligands that are involved in MDSC activation.
MDSC in therapy resistance. Immune checkpoint blockade (ICB) and anti-angiogenic therapies (AAT) are common approaches to tackle malignant disease. Although many patients profit from these therapy regimens, acquired resistance mechanisms limit therapeutic efficacy. Uncovering MDSC-mediated resistance mechanisms is the focus of a translational research project in the lab.

Lisa Hofer
I am a PhD student studying the activation of MDSCs in cancer, driven by a passion for understanding how immune cells adapt in the tumor microenvironment. Outside the lab, I love spending time in nature, preferably while traveling and exploring new places.

Tamina Österreicher
I am curious about the processes underlying immunosuppression in cancer. Laying the focus on myeloid suppressor cells and how they are activated and exert their effector functions, I hope to gather novel findings throughout my PhD. Besides research, I love to explore new travel destinations, spend time outdoors surrounded by good music.

Theresa Schmöger
As a Master student with a focus in the field of immunology, I have always been very interested in cancer research and especially the impact of the tumor microenvironment. I am very excited to work in this field and gain experience in the Oberle Lab for my master thesis. In my free time, I enjoy cooking and baking and follow creative hobbies such as writing and painting.

Manuela Träger
As a trained animal caretaker, I have long-lasting experience in the work with animals and the execution of research experiments with mice. My current role in the lab is to plan and perform mouse experiments and to support the students to guarantee the highest care standards in the performed animal experiments.
- Oberle R, Kührer K, Österreicher T, Weber F, Steinbauer S, Udonta F, Wroblewski M, Ben-Batalla I, Hassl I, Körbelin J, Unseld M, Jauhiainen M, Plochberger B, Röhrl C, Hengstschläger M, Loges S, Stangl H. The HDL particle composition determines its anti-tumor activity in pancreatic cancer. Life Science Alliance, 2022 May 16. PMID: 35577388, DOI: 10.26508/lsa.202101317
- Fritsch SD, Sukhbaatar N, Gonzales K, Sahu A, Tran L, Vogel A, Mazic M, Wilson JL, Forisch S, Mayr H, Oberle R, Weiszmann J, Brenner M, Vanhoutte R, Hofmann M, Pirnes-Karhu S, Magnes C, Kühnast T, Weckwerth W, Bock C, Klavins K, Hengstschläger M, Moissl-Eichinger C, Schabbauer G, Egger G, Pirinen E, Verhelst SHL, and Weichhart T. Metabolic support by macrophages sustains colonic epithelial homeostasis. Cell Metabolism, 2023 Sep 29:S1550-4131(23)00341-8. PMID: 37804836 DOI: 10.1016/j.cmet.2023.09.010
- Bauer R, Udonta F, Wroblewski M, Ben-Batalla I, Santos IM, Taverna F, Kuhlencord M, Gensch V, Päsler S, Vinckier S, Brandner JM, Pantel K, Bokemeyer C, Vogl T, Roth J, Carmeliet P, Loges S. Blockade of myeloid-derived suppressor cell expansion with all-trans retinoic acid increases the efficacy of anti-angiogenic therapy. Cancer Research. 2018 Apr 19. PMID:29674477; DOI:10.1158/0008-5472.CAN-17-3415
- Wroblewski M, Bauer R, Cubas Córdova M, Udonta F, Ben-Batalla I, Legler K, Hauser C, Egberts J, Janning M, Velthaus J, Schulze C, Pantel K, Bokemeyer C, Loges S. Mast cells decrease efficacy of anti-angiogenic therapy by secreting matrix-degrading granzyme B. Nature Communications. 2017 Aug 16. PMID: 28814715; DOI:10.1038/s41467-017-00327-8
- Bauer R, Plieschnig JA, Finkes T, Riegler B, Hermann M, Schneider WJ. The developing chicken yolk sac acquires nutrient transport competence by an orchestrated differentiation process of its endodermal epithelial cells. J. Biol. Chem. 2013 Jan 11. PMID: 23209291; DOI:10.1074/jbc.M112.393090

