
Selma Osmanagić-Myers, Dr.in rer. nat.
BiochemikerinTel.: +43 (0)1 40160-38008
E-Mail: selma.osmanagic-myers@meduniwien.ac.at
Vascular aging and cardiovascular disease
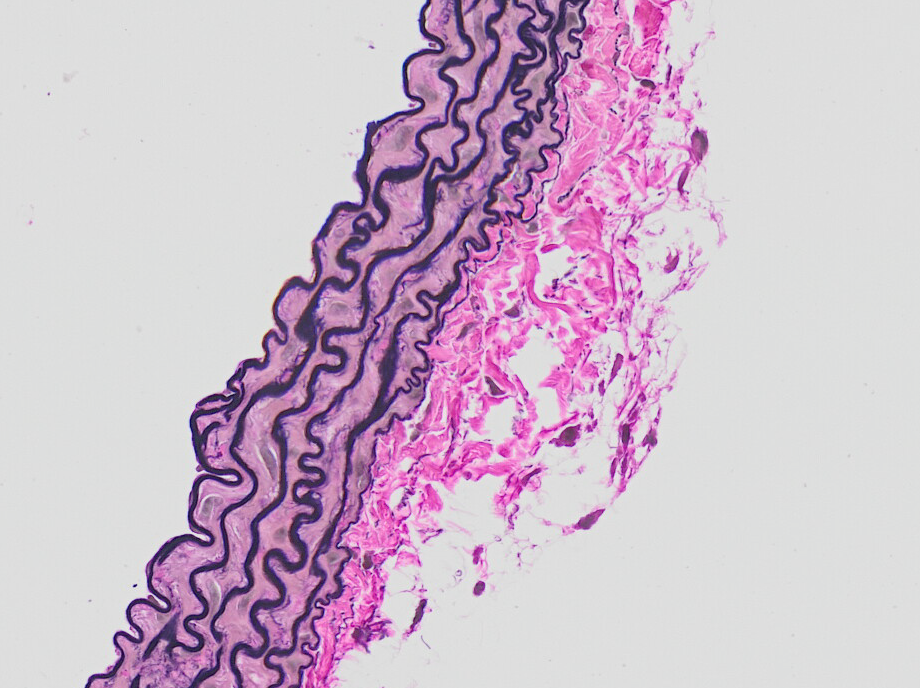
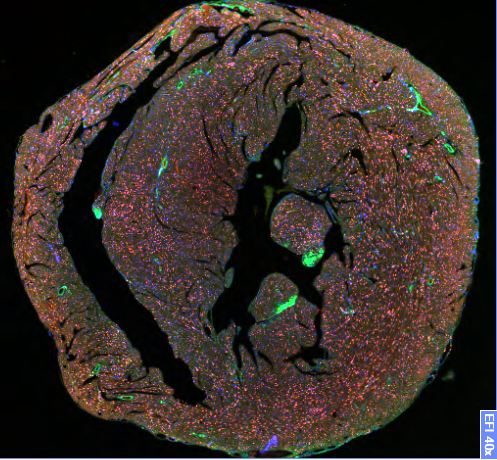
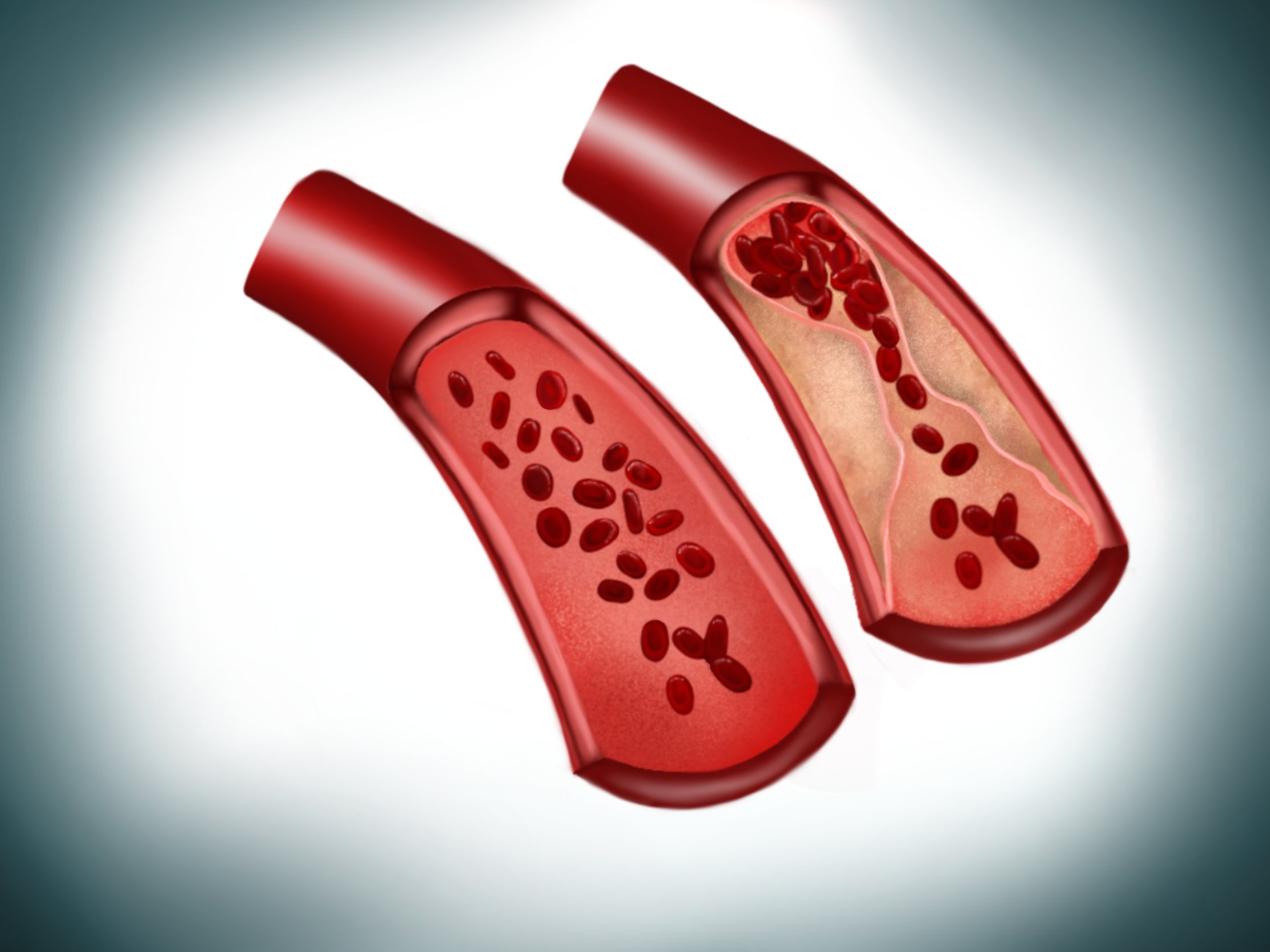
In modern societies with increasingly older populations, age is becoming a major risk factor for cardiovascular disease (CVD)1. However, the underlying molecular mechanisms especially with regard to aging of the innermost blood vessel layer, the endothelium, are still not fully understood. Our major focus is on the impact of cellular aging (senescence) on endothelial dysfunction and development of chronic diseases, particularly, atherosclerosis, cardiovascular disease but also osteoporosis. Currently, we use as a model system our recently generated endothelium-specific progeria mouse that recapitulates major features of accelerated CVD in premature aging disease Hutchinson-Gilford progeria syndrome (HGPS)2. HGPS is caused by a mutation in the LMNA gene and leads to premature death from heart failure at teenage age2-5.
Our recent data on HGPS endothelial cells could show that aging leads to reduced response of endothelial cells to shear stress forces generated by flow of blood or medium over their surface. As a result, endothelial cells secrete profibrotic mediators, members of senescence-associated secretory pathway (SASP), that lead to collagen accumulation in the surrounding tissue, increased cardiac stiffness and ultimately to diastolic dysfunction2.
We aim to further analyze the mechanism of senescence and SASP in endothelial cells and particularly identify endothelial SASP factors in order to work on developing interventions that selectively target these components. Our ultimate goal is to “unwrinkle” the aged blood vessels and promote healthy aging.
Funding
- Childs, B. G., Li, H. & van Deursen, J. M. Senescent cells: a therapeutic target for cardiovascular disease. J Clin Invest 128, 1217-1228, doi:10.1172/JCI95146 (2018).
- Osmanagic-Myers, S. et al. Endothelial progerin expression causes cardiovascular pathology through an impaired mechanoresponse. J Clin Invest 129, 531-545, doi:10.1172/JCI121297 (2019).
- Gordon, L. B., Rothman, F. G., Lopez-Otin, C. & Misteli, T. Progeria: a paradigm for translational medicine. Cell 156, 400-407, doi:10.1016/j.cell.2013.12.028 (2014).
- Osmanagic-Myers, S., Dechat, T. & Foisner, R. Lamins at the crossroads of mechanosignaling. Genes Dev 29, 225-237, doi:10.1101/gad.255968.114 (2015).
- Osmanagic-Myers, S. & Foisner, R. The structural and gene expression hypotheses in laminopathic diseases-not so different after all. Mol Biol Cell 30, 1786-1790, doi:10.1091/mbc.E18-10-0672 (2019).
