
Alexis J. Lomakin, PhD
Junior PITel.: +43 (0)1 40160-38005
E-Mail: alexis.lomakin@meduniwien.ac.at
MECHANOCHEMISTRY OF CELLULAR DECISION-MAKING
Big Picture: The regulatory logic of cell-to-cell heterogeneity in cellular communities
The Lomakin lab asks fundamental questions in biomedical research concerning the origin and functional consequences of phenotypic cell-to-cell variability in a population of genetically identical cells (Fig. 1A). One example of this is so-called phenotypic drug resistance emerging in pathogenic bacteria upon antibiotic treatment or in cancer cells under chemotherapeutic pressure: in response to drugs or other stress stimuli, some cells within the same population survive whereas others die despite the fact that they all have an identical genotype. Yet another example includes populations of epithelial tissue cells that are formed via clonal expansion of single tissue-resident stem cells: while genetically homogeneous, such clonal populations self-organize into a mosaic of phenotypic states including dormancy, active proliferation and migration, as well as terminal differentiation and death, all co-existing in the same community. The phenotypic variability is proposed to prime cells in the same population to respond to differentiation cues and stress signals in a differential fashion, which is crucial for tissue formation, homeostatic turnover, and regeneration upon injury. However, what governs the emergence of phenotypic cell-to-cell heterogeneity in the first place remains an open question. Answering this question is the global objective of our research program.
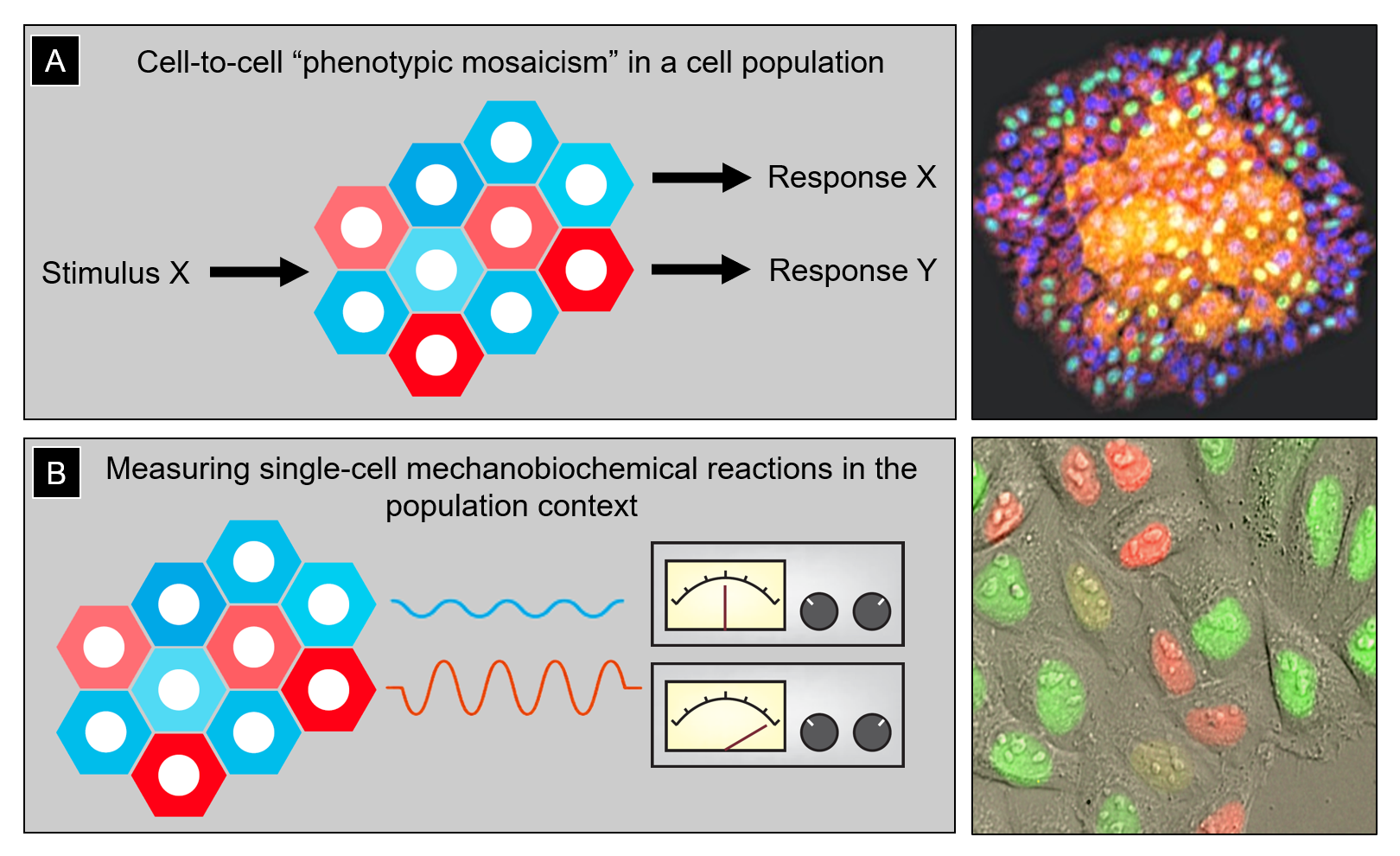
Figure 1: (A) Left, cartoon illustrating cell-to-cell phenotypic heterogeneity in a population of genetically identical cells. This pre-existing heterogeneity determines differential cell responses to the same stimulus (e.g., stress signals or differentiation cues). Right, clonally expanded human epidermal stem and progenitor cells self-organize into a stratified mini-epidermis-in-a-dish in which cells begin to exhibit a variety of phenotypic states with a clear spatial bias (e.g., epidermal progenitors tend to undergo terminal differentiation and stratification in high cell density regions, where cells are more crowded and less stretched). (B) Left, quantitatively assessing differences in mechanobiochemical reactions (e.g., activation of the mechanochemical ATPase myosin II) in individual cells and linking that information to the spatial position of the cells within the population can help infer potential feedback loops between phenotypic cell state and its social context. Right, human cervical carcinoma epithelial cells HeLa-Kyoto expressing the genetically encoded sensor of cell cycle FUCCI (fluorescence ubiquitination cell cycle indicator). Such fluorescent biosensors can be co-imaged with other molecular markers/indicators to account for the inherent variability of cell cycle stages in studies of phenotypic cell heterogeneity.
Our Special Focus: Mechanobiochemistry of cell fate control & tissue patterning
Some of the phenotypic heterogeneity may be caused by the inherent probabilistic nature of biochemical reactions inside a living cell. However, the majority of this variability is not random but forms quasi-deterministic patterns that are regulated. The reason for this is that cells do not operate in isolation but create heterogeneous social contexts to which they adapt their phenotypic behavior. Indeed, spatial and mechanical constraints within cell populations (e.g., local cell density, area of contact with the extracellular matrix and its stiffness, and compression by surrounding tissues) can shape the phenotypic spectrum of constituent cells. Relying on our long-standing interest and expertise in various cell imaging techniques, phenotypic plasticity of epithelial cells, and molecular machines producing mechanical work and sensing physical forces (Lomakin et al. Dev. Cell, 2009; Lomakin et al. Nat. Cell Biol., 2015; and Lomakin et al. Science, 2020), we aim at developing quantitative microscopy-based approaches to capture with a single-cell resolution the mechano- and biochemical readouts of human epithelial stem and cancer cells existing within heterogeneous populations. Mapping those measurements back onto spatial coordinates of cells in the tissue or experimentally manipulating them via cell and tissue microfabrication, we wish to discover quantitative molecular and biomechanical mechanisms through which cells sense their social context and coordinate their phenotype with the spatial position in the population (Fig. 1B). Physics has a great tradition of precision measurement. The lesson that has been learned time‐and‐time‐again is that by measuring fundamental physical phenomena with increasing precision, one can make amazing discoveries and even sometimes stumble across new laws of nature. We hope that by measuring various phenotypic traits of individual cells across different scales of organization and linking them to each other, we will be able to learn how the complex tissue-scale patterns and phenotypic cell variability emerge.
- Lomakin AJ, Cattin CJ, Cuvelier D, Alraies Z, Molina M, Nader GPF, Srivastava N, Sáez PJ, Garcia-Arcos JM, Zhitnyak IY, Bhargava A, Driscoll MK, Welf ES, Fiolka R, Petrie RJ, De Silva NS, González-Granado JM, Manel N, Lennon-Duménil AM, Müller DJ, Piel M. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science. 2020; 370 (6514).
- Lomakin A, Nader G, Piel M. Forcing Entry into the Nucleus. Developmental Cell. 2017; 43: 547-548.
- Lomakin AJ, Lee KC, Han SJ, Bui DA, Davidson M, Mogilner A, Danuser G. Competition for actin between two distinct F-actin networks defines a bistable switch for cell polarization. Nature Cell Biology. 2015; 17: 1435-1445.
- Nadezhdina ES, Lomakin AJ, Shpilman AA, Chudinova EM, Ivanov PA. Microtubules govern stress granule mobility and dynamics. BBA - Molecular Cell Research. 2010; 1803: 361-371.
- Lomakin AJ, Semenova I, Zaliapin I, Kraikivski P, Nadezhdina E, Slepchenko BM, Akhmanova A, Rodionov V. CLIP-170-dependent capture of membrane organelles by microtubules initiates minus-end directed transport. Developmental Cell. 2009; 17: 323-333.
