Markus Hengstschläger, Univ. Prof. Mag. Dr.
Zentrumsleiter, GenetikerTel.: +43 (0)1 40160-56500
E-Mail: markus.hengstschlaeger@meduniwien.ac.at
Human stem cell research to understand genetic disorders
From preimplantation genetic diagnosis and prenatal testing, the discovery of human amniotic fluid stem cells, fetomaternal mircochimerism, the study of self-organization in human embryogenesis via pluripotent-stem-cell-based models of the human embryo (embryoids), and the two potent regulators mTOR and Oct4, to the ethical implications associated with these aspects of research.
Our long lasting involvement in genetic diagnosis in the field of human medical genetics led us to discover stem cells in the human amniotic fluid in 2003 (Prusa, Hum Reprod 2003) and to perform the first preimplantation genetic diagnosis in Austria in 2005 (Hengstschläger, Wien Klin Wochenschr 2005).
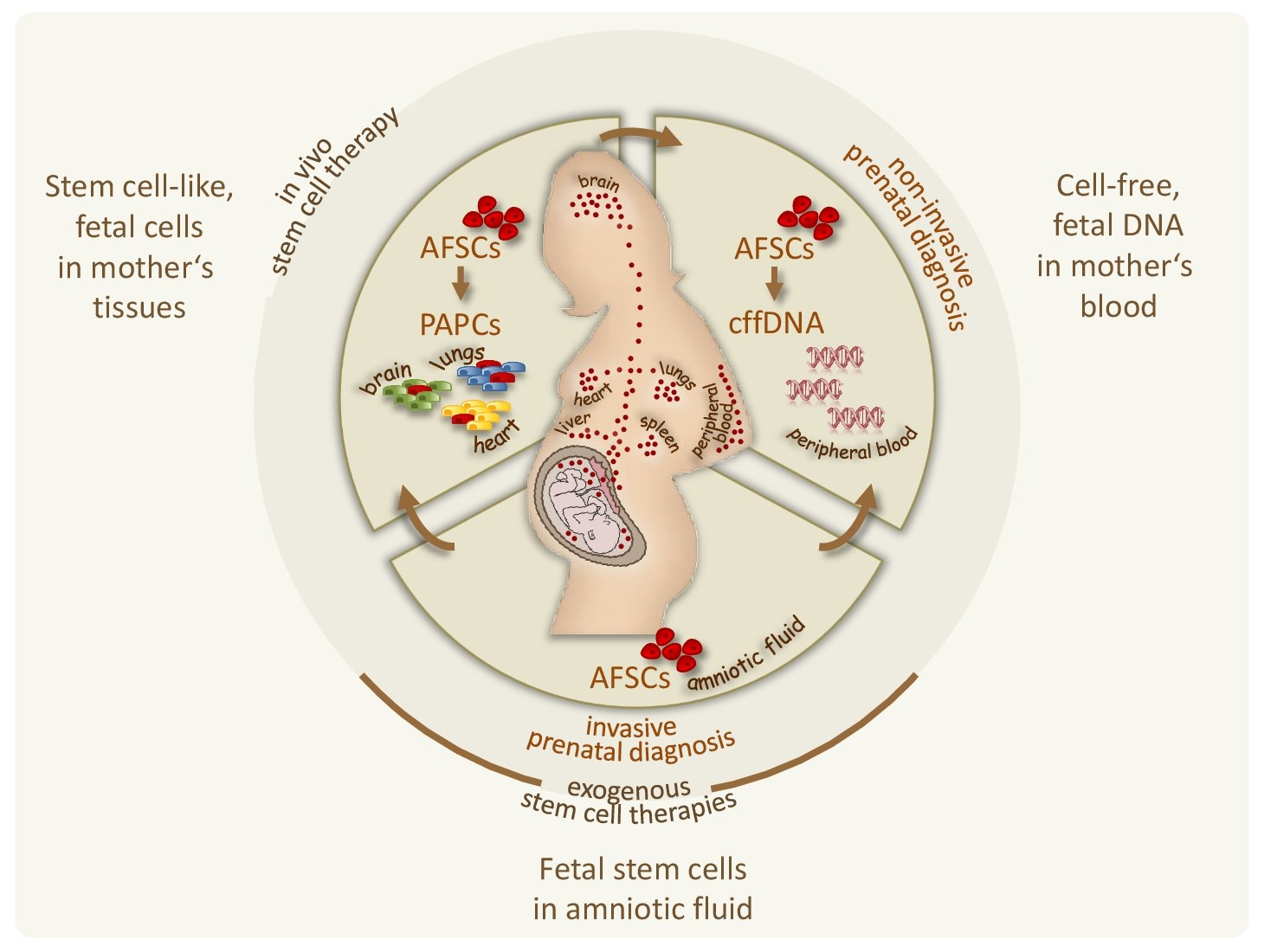
Until now, we are working on the optimization of preimplantation genetic diagnosis approaches (see e.g. Feichtinger, PLos One 2015; Oberle, Clin Chem 2024), and we are investigating the biological role of human amniotic fluid stem cells focusing on their putative involvement in pregnancy-associated fetal cell microchimerism and thus their potential for non-invasive prenatal diagnosis (Fig.1) (see also: Rosner, The New Engl J Med 2012; Rosner, Nat Protoc 2013; Rosner, Mutat Res Rev Mutat Res 2021; Rosner, Stem Cells 2021; Rosner, Stem Cell Rev Rep 2022; Rosner, Clin Chem 2022).
Furthermore, using screening as well as hypothesis-driven approaches and a combination of cell biological, biochemical and computational technologies, our lab investigates how stem cells control their motility and the intertwined parameters of cell cycle, size and survival. We explore the molecular players and the signalling networks which govern these processes, and if or how these differ across cell types. In this context, but not exclusively restricted to stem cell research, we have an already long lasting interest in the Tuberous Sclerosis Complex/mammalian Target of Rapamycin (TSC/mTOR) pathway (see e.g. Soucek, J Biol Chem 1997; Soucek, PNAS 1998; Rosner, J Biol Chem 2004; Freilinger, Oncogene 2006; Weichhart, Immunity 2008; Rosner, Oncogene 2011; Linke, Nat Immunol 2017; Kovacic, Sci Rep 2019).
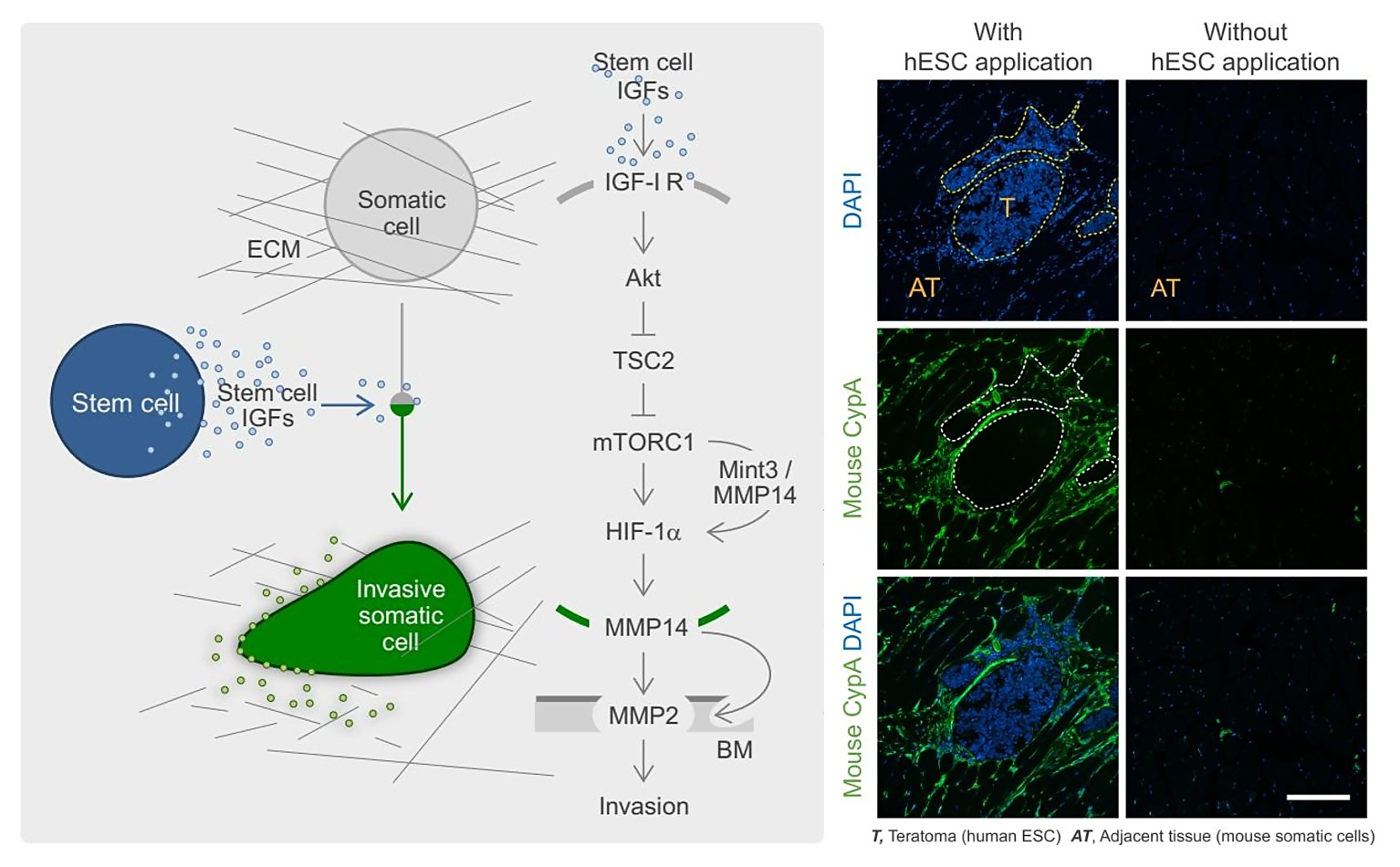
Another key aim is to understand how stem cells communicate with other cells ("the language of stem cells") and how this affects stem cell-related processes such as stem cell tumorigenesis. This includes the identification and characterisation of stem cell-derived paracrine stimuli (Fig. 2) (see also: Rosner, Stem Cells 2016; Rosner, Nat Protoc Exchange 2018).
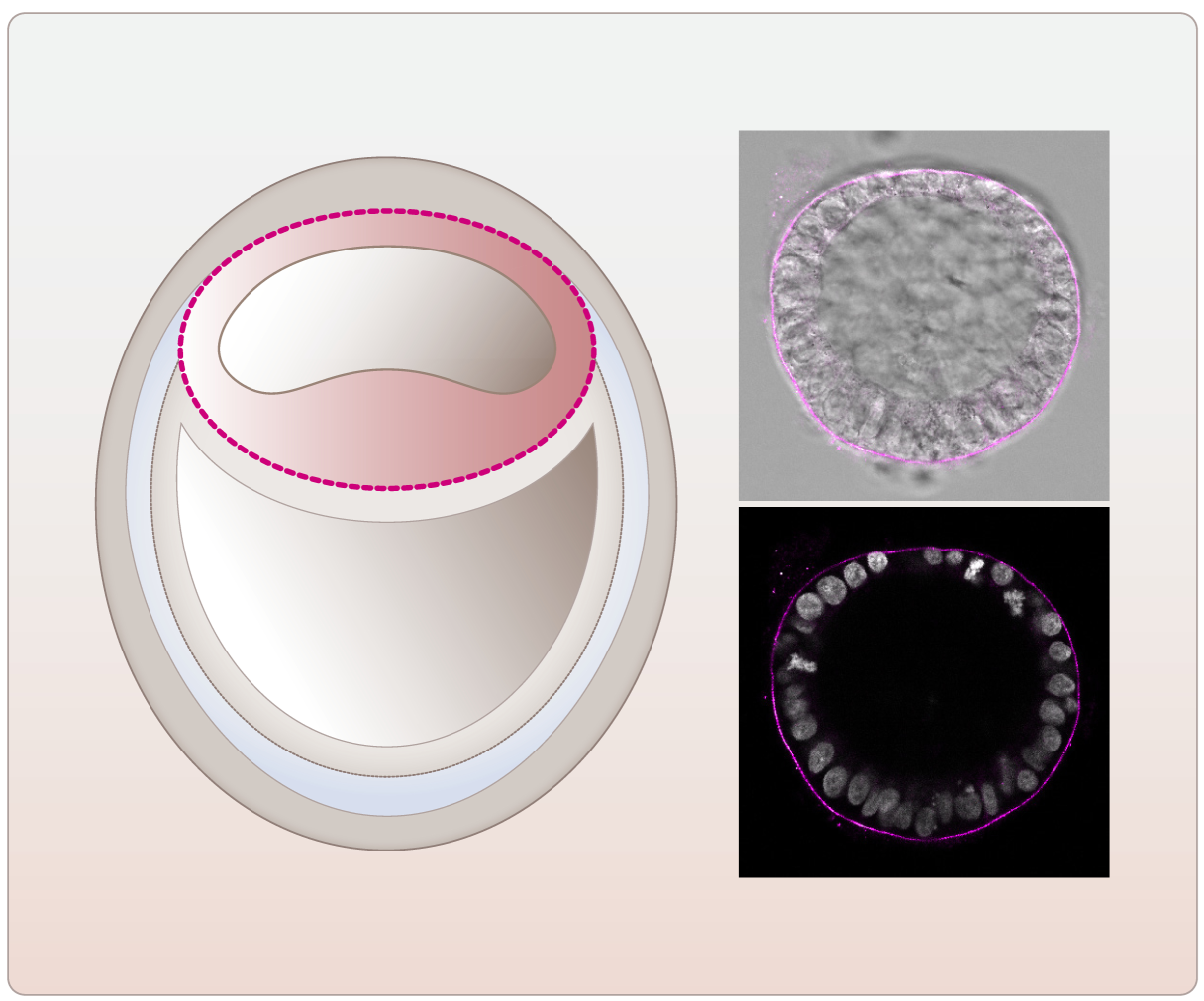
Since many years, we are also working and collaborating on projects making use of in vitro (organotypic reaggregation assays, three-dimensional co-culture models, spheroids, embryoid bodies, organoids) and in vivo approaches to study cell-cell interaction and tissue self-organization (see e.g. Kubista, Oncogene, 2002; Valli, Oncogene 2010; Siegel, Hum Mol Genet 2010; Rosner, Nat Protoc 2010; Unterleuthner, Angiogenesis 2020; Wilson, Cell Reports 2020; Koncina, Nat Comm 2023; Celada, Sci Transl Med 2023; Fritsch, Cell Metab 2023). In this context, we are currently using a pluripotent-stem-cell-based model of the human post-implantation embryo. Up to 50% of human embryos do not develop to the implantation stage or the pregnancies fail, mostly in the first weeks post fertilization. In addition, many human disorders already start to develop during early embryogenesis. However, due to experimental, ethical, and legal limitations and because of the differences between animal and human embryogenesis the regulation of the human peri-implantation period has remained elusive so far. In our lab we mimic this natural human embryonic development including the onset of gastrulation by making use of the stem-cell-based three-dimensional post-implantation amniotic sac embryoid (PASE). Using this embryo model we were able to draw a picture of the assembly of the embryonic basement membrane, to study its disintegration during primitive streak development, and to show that the transcription factor Oct4 is a potent regulator of basement membrane development during human embryogenesis (Fig. 3).
The fact that many of the aspects we are working on in clinical genetics and basic science have ethical implications, prompted us to become involved in this field. Since many years, we are doing research on the ethical implications of prenatal diagnosis, preimplantation genetic diagnosis, exome and genome sequencing, predictive genetic testing, gene therapy, human embryonic stem cells, in vitro fertilization, in vitro gametogenesis, human embryo models, and artificial intelligence in medicine (see e.g. Hengstschläger, Stem Cell Res Ther 2021; Rosner, Stem Cell Res Ther 2023; Horer, Stem Cells Transl Med 2023; Hengstschläger, Hum Reprod Open 2023).