Mario Mikula, Assoc. Prof. Priv. Doz. Mag. Dr., MAS
Geneticist and ToxicologistTel: + 43 (0)1 40160-56540
E-Mail: mario.mikula@meduniwien.ac.at
Research Focus
Differentiation programs active during melanoma development
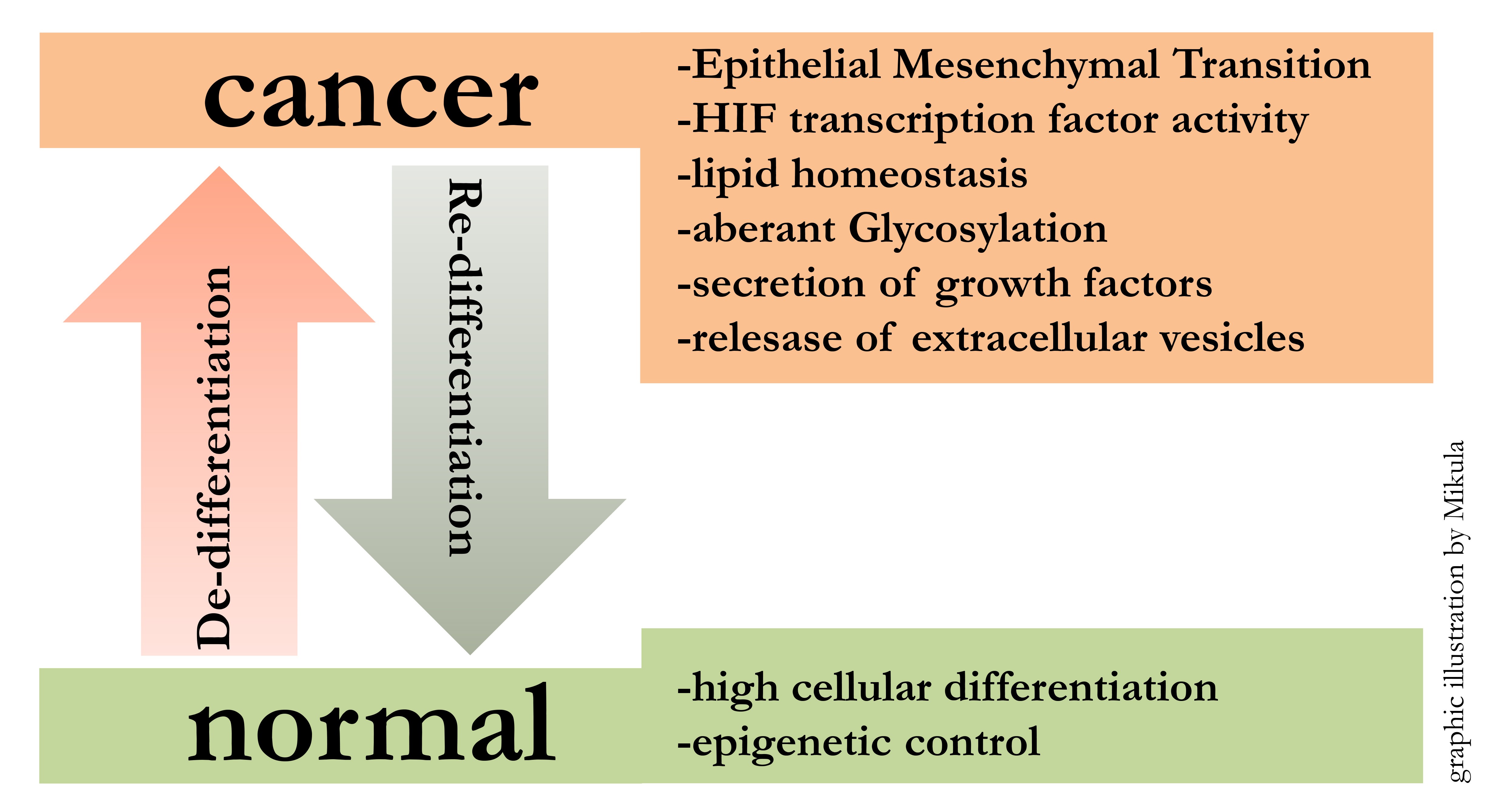
Starting from embryonic stem cells, developmental programs lead to the formation of all organs in the human body. This implies that cellular differentiation mechanisms are powerful determinators of cellular fate. The reactivation of these conserved developmental programs, like EMT (Epithelial Mesenchymal Transition), during and after pathologic events, has profound impact on the outcome of disease. Focussing on cancer, de-differentiation along with tumor progression can be observed. In particular our research has shown that melanoma cells loose differentiation and at the same time gain cancer specific characteristics.
The Mikula Lab focuses on elucidating mechanisms for metastatic melanoma spread (1). Specifically, we are interested in the involvement of an epithelial mesenchymal switch in the process of tumor progression. So far we have studied several signaling pathways in melanoma, which were associated with the EMT phenotype and hypoxia (2, 3), NRF2 (4) or STAT3 (5).
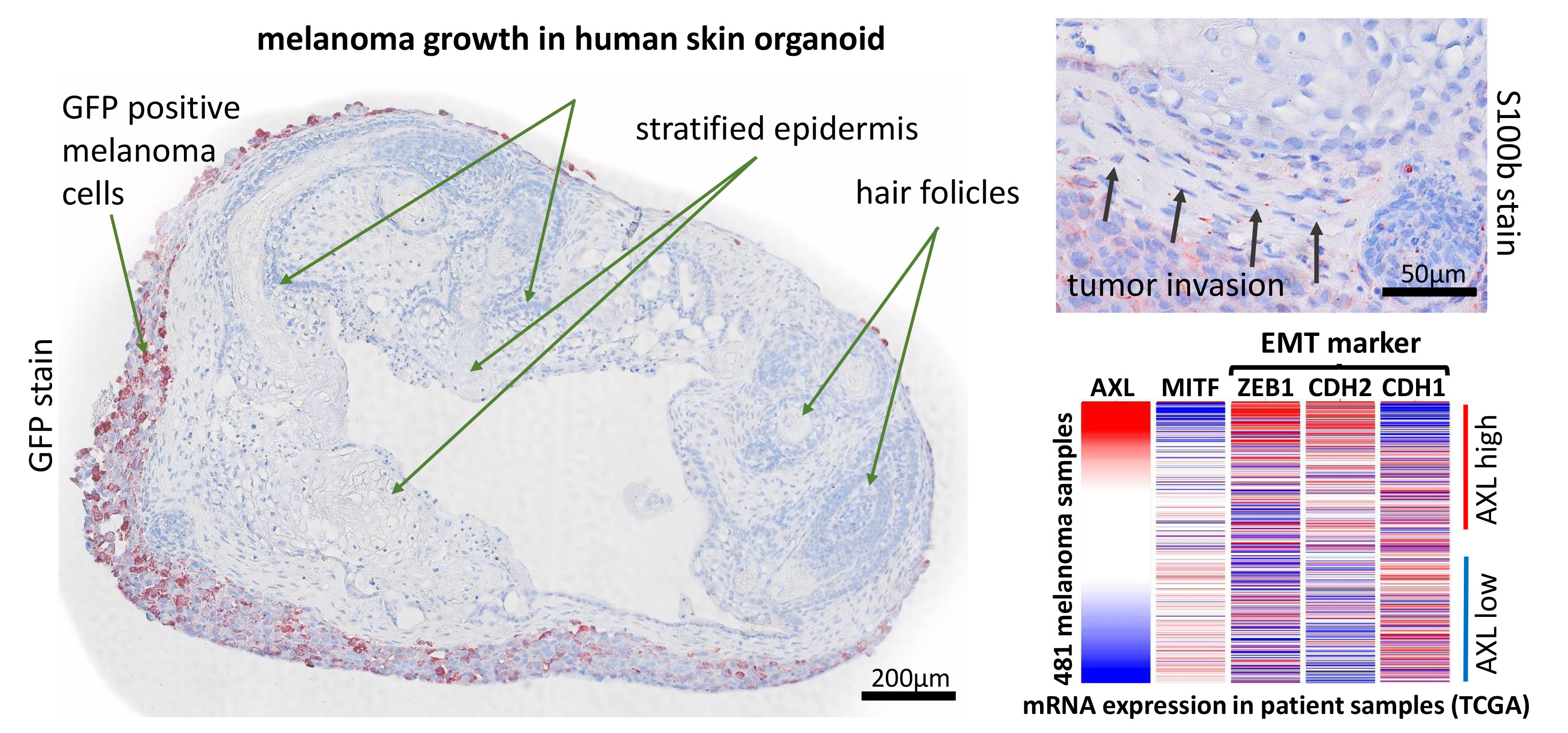
We start a new project to investigate the impact of the AXL receptors in modulating melanoma progression AXL receptor and its ligand GAS6 is connected to the EMT pathway in melanoma and we hypothesize that controlling inhibiting AXL signaling will reduce melanoma invasiveness and metastasis. Hence, this new project will aim to generate and characterize melanoma lines harboring either a deletion (by CRISPR-Cas9) or overexpression (viral) of human AXL receptor. Furthermore, we will make use of a novel human pluripotent stem cell-derived skin organoid model (6), which can be used as a host for melanoma cells. With the help of this model we will monitor tumor growth, invasion of adjacent tissue and marker expression associated with cell cycle, apoptosis and differentiation. Additionally, we will generate iPS cells from genetically modified melanoma, which are subsequently used to form pure or chimeric skin-organoids, which are expected to give rise to in situ melanomas. Outcome will be measured by live microscopy imaging, histologic analysis and cell sorting, followed by mRNA quantification.
We envision that this project will be embedded in our current research efforts (3 PHD students, 1 research assistants). The project will be carried out in a stimulating work environment with access to advanced methods (Histolab in house, Leica TCS SP8 and Nikon spinning disc, Cytoflex S, Sony cell sorter, NGS …) and sufficient research funding.
References
1. Mirea, M. A., S. Eckensperger, M. Hengstschlager, and M. Mikula. 2020. Insights into Differentiation of Melanocytes from Human Stem Cells and Their Relevance for Melanoma Treatment. Cancers (Basel) 12.
2. Kinslechner, K., D. Schorghofer, B. Schutz, M. Vallianou, B. Wingelhofer, W. Mikulits, C. Rohrl, M. Hengstschlager, R. Moriggl, H. Stangl, and M. Mikula. 2018. Malignant Phenotypes in Metastatic Melanoma are Governed by SR-BI and its Association with Glycosylation and STAT5 Activation. Mol Cancer Res 16: 135-146.
3. Kinslechner, K., B. Schutz, M. Pistek, P. Rapolter, H. P. Weitzenbock, H. Hundsberger, W. Mikulits, J. Grillari, C. Rohrl, M. Hengstschlager, H. Stangl, and M. Mikula. 2019. Loss of SR-BI Down-Regulates MITF and Suppresses Extracellular Vesicle Release in Human Melanoma. Int J Mol Sci 20.
4. Weitzenbock, H. P., A. Gschwendtner, C. Wiesner, M. Depke, F. Schmidt, F. Trautinger, M. Hengstschlager, H. Hundsberger, and M. Mikula. 2022. Proteome analysis of NRF2 inhibition in melanoma reveals CD44 up-regulation and increased apoptosis resistance upon vemurafenib treatment. Cancer Med 11: 956-967.
5. Swoboda, A., R. Soukup, O. Eckel, K. Kinslechner, B. Wingelhofer, D. Schorghofer, C. Sternberg, H. T. T. Pham, M. Vallianou, J. Horvath, D. Stoiber, L. Kenner, L. Larue, V. Poli, F. Beermann, T. Yokota, S. Kubicek, T. Krausgruber, A. F. Rendeiro, C. Bock, R. Zenz, B. Kovacic, F. Aberger, M. Hengstschlager, P. Petzelbauer, M. Mikula, and R. Moriggl. 2021. STAT3 promotes melanoma metastasis by CEBP-induced repression of the MITF pathway. Oncogene 40: 1091-1105.
6. Lee, J., C. C. Rabbani, H. Gao, M. R. Steinhart, B. M. Woodruff, Z. E. Pflum, A. Kim, S. Heller, Y. Liu, T. Z. Shipchandler, and K. R. Koehler. 2020. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 582: 399-404.
Links
We have been or are funded by the following agencies:
Herzfeldersche Familienstiftung
Role of FGF signaling in Melanoma
Austrian National Bank
Reactivation of Embryonic Pathways in Melanoma
Austrian Science Fund FWF
FWF(DFH 44-B): Transformation of preclinics to clinics by organoids (TOPICO)
FWF (P35387-B): Human skin organoids, a novel host for cancer grafts
FWF (P32979-B): Identification of a novel HIF regulation in human melanoma
FWF (P 25336-B13): The Lipogenic Phenotype in Melanoma
Austrian Research Promotion Agency FFG
FFG (841291): Generation of a metastasis specific antibody.